
"Chemistry"...For many, this word often conjures up an image of a mad scientist mixing substances in a cluttered laboratory in which hundreds of flasks gather dust on the shelves. This image often overshadows the fact that chemistry is a strict science which studies the constitution of matter. It focuses on atoms• — which are assembled together in an organized and structured fashion to form molecules• — as well as all the mechanisms, processes and interactions in which they are involved. Moreover, chemicals are often assimilated with hazardous substances. This is not necessarily the case. All matter is made up of chemicals or a mixture of chemicals.
The word chemistry is derived from the Arabic al-kimi alchemy”, which was borrowed either from the Greek khumeia “pouring together”, or from the Egyptian khemet meaning “earth”. Chemistry was therefore originally the art of preparing, purifying, transforming and utilizing naturally occurring substances.
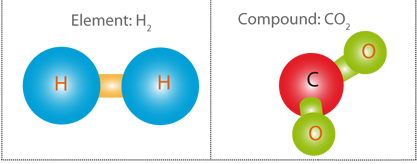
From a scientific point of view, chemicals may be pure substances made up of a single type of component, or mixtures. Pure substances can be elements (i.e. composed of a single type of atom) or compounds (i.e. composed of different atoms).
From an industrial point of view, chemicals are defined
according to their place in the production chain. The
following categories can be distinguished:
To find out about the scientists who have transformed chemistry from antiquity to the present day, see the website: www.chemheritage.org/
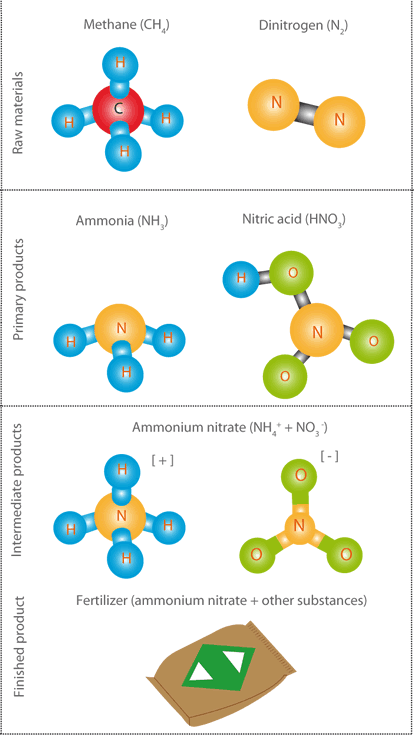
This document will mainly focus on raw materials, primary products and intermediate products.
In 1869, the Russian chemist Dimitri Mendeleev designed a table presenting all the chemical elements identified at the time in an orderly manner. Since then, this table has undergone many changes.
In 2016, it comprised 118 elements sorted according
to their atomic number, i.e. their number of protons• (positive charges).
Each column is made up of elements with similar physical and chemical properties. Each row comprises elements with a similar electron• (negative charges) arrangement around the nucleus•.
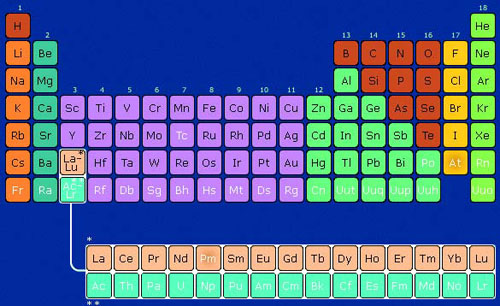
An interactive version of the Periodic Table can be found on the elements.wlonk.com website:
http://elements.wlonk.com/ElementsTable.htm
Last update: 8/11//2016