Raw materials are extracted from naturally occurring deposits in the environment.
Solid materials are extracted from mines or quarries.
The ground is dug up and the fragments of rock recovered are sorted. Deposits containing a large quantity of the material sought are selected first. This is the case for copper•, rock phosphate•, iron•, coal•, lead• and salt.

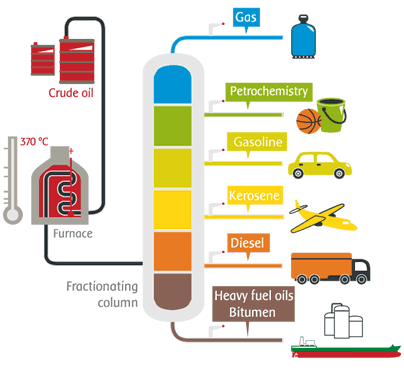
Liquids and gases accumulate in pockets in bedrock, and can only be extracted by drilling and introducing suitable pipes. This is the case of crude oil and natural gas (e.g. methane•).
Many primary or intermediate products are obtained from a series of processes gathered under the term "synthesis". This involves the processing of several pure substances, through a number of reactions•, in order to produce the desired products. Synthesis is employed in many industries such as:
the food industry (flavourings), the pharmaceutical industry (medicines), the cosmetic industry (soaps, perfumes).
Some primary or intermediate products can be obtained by separating elements from a mixture. The three main types of industrial processes used in this case are distillation, evaporation and filtration.
During distillation, the mixture is heated until the
products evaporate. They are then recovered separately by vapour condensation.
Evaporation is employed to separate a volatile solvent• from a non-volatile liquid phase• in which it is in solution.
Filtration is used to separate components from a
mixture, through a selective filter.