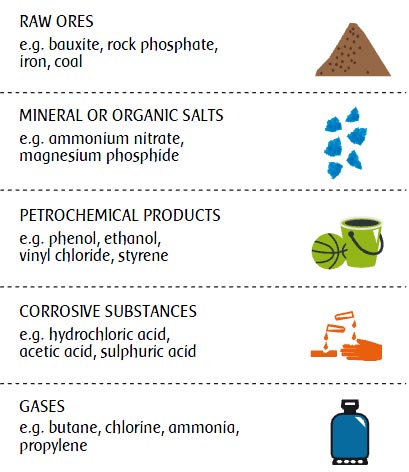
Chemicals can be classified according to extremely varied criteria such as: their molecular structure, their behaviour, the hazards they present... giving rise to complex classifications. Nevertheless, five broad families can be distinguished based on the chemicals’ nature:
IUPAC (International Union of Pure and Applied Chemistry)
nomenclature: find out about the rules in force on the nomenclature of organic compounds at:
http://www.chem.qmul.ac.uk/iupac/
Industry Canada, a source of information on the chemical
industry in Canada and abroad:
www.ic.gc.ca/eic/site/chemicals-chimiques.nsf/eng/home/
Organic chemistry by definition covers
all that relates to the living and concerns the description and study of a vast
range of mainly carbon-based molecules.
Its scope has slightly expanded over recent years to include the field of
chemistry that aims to synthesize molecules
composed exclusively of carbon, different to those found in nature.
In addition to these naturally occurring or synthetic carbon molecules, organic chemistry includes molecules containing
elements such as hydrogen, oxygen, nitrogen, phosphorus, sulphur•, and halogens.
The purpose of inorganic chemistry, formerly known as mineral chemistry, is to study molecular substances that are
not of biological origin. It particularly focuses on minerals (e.g. quartz, aluminosilicates, cements), mineral acids
(e.g. hydrochloric acid), metals (e.g. iron, aluminum•, mercury) and alloys.